Discover the future Dentistry today
Dental Medical Integration
...because
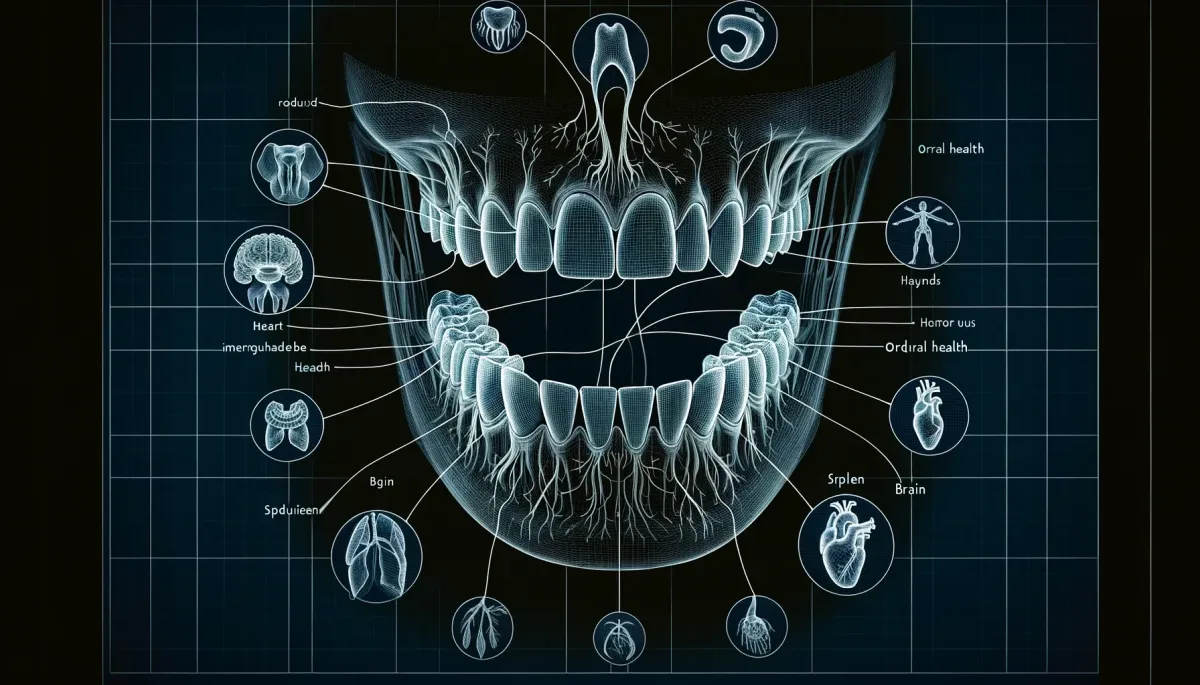
health starts from the mouth

Medical-Dental Integration
Improve Patient Care

Increase Revenue

Healthy Oral Health Care

Protect Your Practice
Get More New Patients Easily
Download your Guide
Upgrade Your Dental Practice
Get the insider guide to be like the top 1% of dental practices
OUR MISSION
Get Dentists Paid more for the work they already do so they can help as many patients that need their help as possible
Let's end dental-medical segregation together forever
Our Packages
Professional
two day custom training course
Premier
one day custom training course
Online Course
self-study course
100% FREE DENTIST CASE STUDY
HOW WE ADDED $2,681,746.29 WITH 862 PATIENTS FOR A DENTAL PRACTICE IN CALIFORNIA
Get the Case Study to See What Medical Integration Can Do for Dental Practices
Discover
Dental done right
You should get recognized and paid for the "Doctor Of" portion of your license and work you do as a Dentist, don't you agree?

